
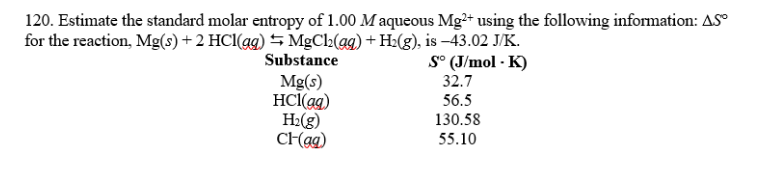
#Molar entropy trial
In vivo efficacy trial illustrated that CPP−1 supplements could alleviate HFD-induced mice obesity significantly, as well as improve obesity-induced disorders of glucose metabolism, alleviate insulin resistance, and improve the effects of lipid metabolism. The methylation analysis revealed that the main glycosidic linkage types of CPP−1 were (1→)-linked-Glc residue, (1→3)-linked-Glc residues, (1→4)-linked-Gal residue, (1→2,3,4)-linked-Glc residue, (1→)-linked-Man residue, (1→3,4)-linked-Glc residue, and (1→)-linked-Ara residue. The results indicated that CPP−1 was composed of mannose (Man), glucose (Glc), galactose (Gal), and arabinose (Ara) at a molar ratio of 5.86 : 51.69 : 34.34 : 8.08. Unit of Entropy (ue, eu) has a dimension of ML2T-2Q-1N-1 where M. Its structure was analyzed by HPGPC-ELSD, HPLC, GC-MS, FT-IR, and NMR techniques. The resulting operational equation for the calculation of the molar entropy of the substance at the temperature and pressure of interest is (6.2.2) S m ( T ) S n 0 T C p, m T d T + trs H T trs (pure substance, constant p) where C p, m C p / n is the molar heat capacity at constant pressure. Compute thermodynamic properties such as entropy, heat capacity or vapor pressure for a wide variety of chemicals. Unit of Entropy (ue, eu) is the only unit in the category of Molar entropy in our database. by water extraction, ethanol precipitation, and column chromatography. This convention produces negative standard entropies for some ions. With the transition state defined as above, its standard absolute entropy Sis is. pressure 1013.25 hPa and temperature 25 C. Adsorption entropy Previously, we introduced the common first-order. In addition, the standard molar entropy of the hydrated proton is taken as zero 5 (H+, aq) 0. Standard entropy of the substance refers to so-called standard conditions i.e. 1) The standard molar entropies of reactants and products of a reaction can be used. For any element in its standard state, the value of entropy has a definite, non-zero value. A homogeneous polysaccharide coded as CPP−1 was extracted and purified from the root of Codonopsis pilosula (Franch.) Nannf. Conventional values of the standard molar Gibbs energy of formation and standard molar enthalpy of formation of the hydrated proton are ArC (H +, aq) 0 and Ar// (H +, aq) 0. The standard molar entropy is the total (minimal) amount of entropy gained by 1 mole of a substance when its temperature is increased from 0K to the standard conditions.


 0 kommentar(er)
0 kommentar(er)
